The Mystery of the Variants
The Mystery of the Variants
A new preprint on recent evolution in the SARS-2 spike protein raises bizarre questions.
While the press narrative on Variants of Concern involves massive, manipulative exaggeration, there is a real phenomenon at the heart of it. The SARS-2 genome, particularly the portion that codes for the S1 subunit of spike, began experiencing extreme evolutionary pressure between June and October 2020. Nobody knows why, but this much is clear: The variants – including the Delta strains that destroy the effectiveness of our vaccines – arose as SARS-2 responded to this pressure. You could almost say that the virus began to escape our vaccines, before anybody outside of the trials had been vaccinated.
This is the bizarre story that emerges from “Rapid and parallel adaptive mutations in spike S1 drive clade success in SARS-CoV-2”, an August preprint from researchers in Washington on SARS-2 evolution over the past year. See also this video by Trevor Bedford, one of the authors, summarising the same findings. Nearer our sphere, there is this in-depth analysis from Gigaohm Biological and these remarks from the Nepetalactone Newsletter.
For the first months of the pandemic, SARS-2 demonstrated remarkable stability. Mutant strains emerged, as they always do, but they did worse than non-mutant strains and were generally out-competed. This is what you would expect of a virus that had been optimised to infect human lung tissue in a laboratory. The only major mutation to mention, D614G, was first noticed in mid-January 2020. Strains with this mutation beat out all other lineages, which is the best proof possible that it conferred enhanced transmissibility.
The relevant slide from Bedford’s video presentation:
You might say this was a Variant of Concern avant la lettre, before it had occurred to anyone to pay attention to Variants of Concern. Before this mutation, we find suggestions here and there that SARS-2 had slightly different characteristics. The 27 February Report of the WHO-China Joint Mission on Coronavirus Disease 2019, written before the D614G had achieved wide dominance, has relaxed views of SARS-2 infectiousness. Its authors discount the possibility of aerosolised transmission, insisting that the virus “is transmitted via droplets and fomites during close unprotected contact between an infector and infectee” and that “Airborne spread has not been reported.” They go on to say that “In China, human-to-human transmission of the COVID-19 virus is largely occurring in families” and that they “estimate the secondary attack rate in households ranges from 3-10%.” The ceiling of this range – 10% – is where the secondary household attack rate for Delta strains sits right now, according to Public Health England data.
So there’s a few early changes, but SARS-2 doesn’t experience a huge wave of adaptations right away, as you’d expect from a normal zoonotic event. The virus is fine-tuning its abilities in early 2020; it’s not struggling to make itself at home in its new human hosts, because it has been prepared already for human hosts.
In this light, our spike-focused vaccine strategy begins to make sense. The idea of targeting the Coronavirus spike protein with viral vector (or mRNA) vaccines predates the Corona pandemic; MERS vaccine developers have for years plotted a spike-specific vaccine, both for humans and for camels, the major animal reservoir. But you can see how this approach must have seemed even more compelling in the context of SARS-2. Everyone more or less knew this virus had already been enhanced by gain of function research. Their theory would’ve been that spike had nowhere to go but down. That is, the virus might evolve to evade spike-specific vaccines, but it could only do so by mutating in sub-optimal ways that hurt its binding affinities.
It turns out that this was a bad theory.
Just as the vaccines entered Phase 3 trials, the S1 subunit of spike began to show signs of rapid, adaptive change in multiple locations across the world. The virus went from wanting nothing so much as to keep spike the way it was, to wanting a rather different and new spike protein at all costs.
Genomes are instructions for building proteins. Just like there are many different ways to build the same Ikea desk, there are many different ways to code for the same protein. Synonymous mutations are changes to the genetic code that don’t influence the amino acid that they code for. These are evolutionarily meaningless mutations, because they don’t affect the virus itself. Nonsynonomous mutations are the opposite. They are changes to the genetic code that result in different amino acid sequences and thus different proteins. By the ratio of nonsynonmous (significant) to synonymous (silent or insignificant) mutations, we can get a handle on what kinds of pressure the virus is under. If there are fewer nonsynonomous that synonymous mutations, that means the virus is experiencing purifying or negative selection. Changes to its proteins are disfavoured, and mutations are weeded out. But if there are more nonsynonymous mutations, then it’s the opposite: It means that mutant strains are favoured, while the conservative strains with the old protein sequences face a disadvantage.
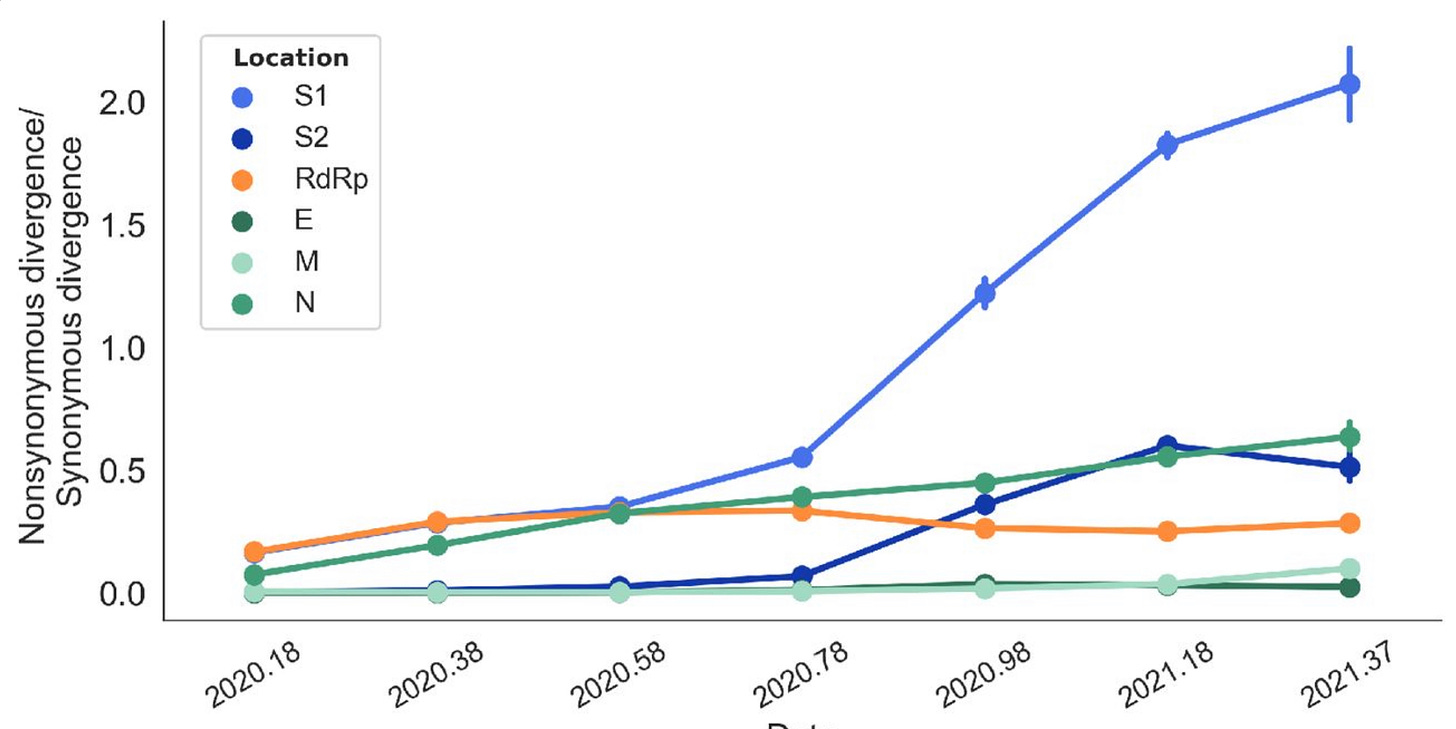
With that in mind, behold this chart, which plots the ratio of nonsynonymous to synonymous mutations over time for different components of SARS-2. The crucial S1 protein is the blue line:
“2020.58” on the x-axis is 58% of the way through 2020, corresponding to a date around the end of July 2020; “2020.78” is around 10 October. You can see that, precisely within this 70-day period, S1 begins to experience runaway and highly unusual positive selection pressure. Until that moment the virus was stable. Thereafter, we start get variants.
The preprint authors establish that these variants are a mix of two things: They carry some mutations that enable the virus to escape immunity, and other mutations that enhance SARS-2 transmissibility. These changes are convergent across multiple lineages: “[S]ome of the notable mutations that have occurred multiple times independently … appear in multiple [Variants of Concern].” This is the classic sign of a mutation that confers substantial advantage. And then there is the fact that “Substitutions within S1 cluster temporally.” In other words, the variant mutations don’t crop up at a steady pace over many months. Instead, they happen in short bursts and in combination with one another.
The big, big question, is simply this:
What is happening in late summer or early fall 2020 that drives the SARS-2 spike protein to evolve so rapidly?
It’s a mysterious problem. The pressure is felt only on S1. Other virus proteins remain more or less unaffected.
Let’s go through a few theories.
First, there is the orthodox suggestion of the researchers themselves:
This temporal structure likely indicates a changing evolutionary landscape: either through the emergence of new selective pressure, and/or through the occurrence of permissive mutations that made adaptive mutations more accessible.
In the YouTube video, Bedford furthermore suggest that longer, persistent infections in people with “partial immunity” may favour the kinds of complex evolution necessary for the emergence of these mutant variant strains. He needs “partial immunity” to be part of the equation, because otherwise, he can’t explain why this evolution didn’t happen in the course of the millions of infections prior to July 2020.
Our current Variants of Concern are more than just one mutation, like D614G from January 2020. They’re more like 8 mutations, none of which are all that advantageous by themselves. One or two of these aren’t enough to make much difference, and so it’s very unlikely any single mutant strain will acquire all eight by chance. Perhaps, though, if you have a long, persistent infection, with partial immunity, you start cultivating multiple mutant strains within your body. Maybe one of these strains picks up mutations 1-2 of the 8 necessary; another one picks up mutation 3; and then through recombination events (which occur when the same cell is infected by different strains), you get a combined strain with 3 of the 8 mutations. And meanwhile you’re still sick, and the virus is trying out still more mutations. In this way, a weak immune response might actually end up training the virus.. Before July 2020, Bedford would say, you didn’t have enough persistent infections driven by partial immunity for this scenario to reach critical mass, so S1 remained more stable.
The problems with this theory seem obvious: There had been cross-immunity in the population from prior exposure to other Coronaviruses long before SARS-2 ever emerged. Wouldn’t that have caused an analogous effect in the first wave? And why is the effect seen only on the S1 subunit of spike? The natural immune response targets multiple virus proteins. And above all: Why is the effect so extreme?
The dissident view argued by the guys I linked up top is that the vaccines are involved somehow. This would seem a powerful explanation for the runaway pressure on S1. Our vaccines, after all, confer lopsided immunity primarily against this singular subunit of spike. The problem is that this effect emerges too soon, after July 2020, at a point when almost nobody anywhere has been vaccinated. The argument would have to be that it is the vaccine trials that caused this effect, via conferring “partial immunity” on their participants. I confess that I find this unconvincing. The trials involved very few people, a vanishing minority of whom can have contracted SARS-2 between July and October 2020. Even if we assume a very high undetected case rate in the vaccinated arm of these trials, we just can’t be talking about more than a few hundred infections. Maybe you could push that number to a thousand if we take all the Phase III trials together. It’s hard to imagine how this could represent a significant addition to the pool of persistent infections in the partially immune happening across the whole world already. If the vaccines are implicated, I don’t think this can be the way.
Conspiratorial possibilities, of course, abound: Maybe mass vaccinations were undertaken secretly in the summer of 2020, perhaps via aerosolised adenovirus vector vaccines, or aerosolised spike proteins – the very same technology that EcoHealth Alliance, in partnernship with the Wuhan Institute of Virology, proposed to use on bats in 2018. Maybe this is how China really beat SARS-2. Alternatively, you could imagine that whoever was behind the release of the virus in the first place began releasing updated variants to defeat the vaccines sometime around July.
These scenarios are offered for your entertainment more than your serious consideration. The only really important point to take away from all of this, is that mass vaccinations targeting the SARS-2 spike protein were a tremendous strategic blunder. Even before these vaccines could be deployed, their protein target was running away from them. As early as Fall 2020, the Delta branch that would yield our current escape strains was already circulating.

Comments
Post a Comment