The First Tyson/Fareed Study Text
The First Tyson/Fareed Study Text
Understand that I say "first" because I don't know that I won't just volunteer my time to sort through the rest of their data when there is a good moment. However, it feels like the world is moving too fast for now, and I can work nearly every waking hour and not come close to keeping up with the pace.
Brian's and George's story is out, and the decision was made in December to include in the book the study I worked up and wrote for them primarily around April-May of 2021. I'm also going to post it here at Rounding the Earth so that everyone who wants to read it can do so freely. Science should not be gated.
Treat this as a preprint, which is to say that changes can be made where commenters convince us to make changes. We may soon upload it to a preprint server, though publication is less the point than presentation for the public.
Low Rates of Hospitalization and Death in 4,376 COVID-19 Patients
Given Early Ambulatory Medical and Supportive Care.
A Case Series and Observational Study.
Contributors to the study:
Brian M. Tyson, M.D., George Fareed, M.D., Emmanuel Beltran Guiterrez, M.D., Robert Villegas, NP, Edgar Josue Anaya Gomez, M.D., Paloma Serrano Lopez, M.D., Ernesto Breton Herrera, M.D., Jesus Palomera III, M.D., Christiany Alexandrah Morales, Ana Mariella Escutia Gonzalez, Fabiola Tyson, NP, M.D., Mathew Crawford
Address for Correspondence
Mathew Crawford
mathew.crawford@protonmail.com
Word Count: 4046
Funding source(s): self-funded
Conflict of interest statement for author: nothing to disclose
Authors had access to the data and wrote the manuscript
Running head: Outcomes after Ambulatory Treatment of COVID-19
Abstract
Background: This study reports the results of early ambulatory protocols in treating 4,376 COVID-19 patients at All Valley Urgent Care (AVUC) facilities in Imperial County, CA, and compares those treatment results to outcomes of other patients in the same county during a nearly identical time period, and encourages a better framework for analysis of such results while adding to the growing body of evidence as to whether such treatment reduces the need for hospitalization and lowers mortality rates. The broad application of face-to-face ambulatory treatment of SARS-CoV-2 infection has not broadly been undertaken in the United States.
Methods: We examine the results of two similar multidrug protocols with data from the immediate county. Protocol 1 uses of hydroxychloroquine, an agent with apparent antiviral reactivity against SARS-CoV-2, two antibiotics (azithromycin, doxycycline), along with a multivitamin pack (including zinc, vitamin C, vitamin D, and others), and with selective use of one or a combination of inhaled budesonide, dexamethasone, prednisone, or other treatments deemed appropriate. Protocol 2 includes all of these options, plus ivermectin where deemed appropriate by physicians. Results were then stratified according to disease severity of patients when first seen by doctors. Among 4,385 individuals sorted in both protocols and three severity levels, combined or excluded from this study, the mean age was 40.5±18.2 years and 12.8% were less than twenty years of age.
Primary Results: Among 3,962 patients treated for mild COVID-19, prior to the development of moderate or severe levels of disease stage, none died as compared to 3.03% (2.25% risk adjusted) (OR = 0.0000, p < 0.0001) in the same county and time period. Of those 3,962 patients, 0.05% were hospitalized as compared to 22.68% (20.76% risk adjusted) (OR = 0.0019, p < 0.0001) in the same county and time period. Differences in outcomes among All Valley Urgent Care patients based on the severity at the time of presentation were observed. Those patients receiving treatment while presenting mild COVID-19 were less often hospitalized (OR = 0.0293, p < 0.0001) and suffered lower mortality (OR = 0.0000, p = 0.0008). Results suggest a highly significant impact for these multidrug protocols. No serious side effects, including cardiac side effects, were observed.
Key words: SARS-CoV-2; COVID-19; multidrug; hospitalization; mortality; ambulatory; antiviral; zinc; hydroxychloroquine; ivermectin; doxycycline; azithromycin; vitamin; corticosteroid
1. Introduction
The epidemic viral outbreak of SARS-CoV-2 infection (COVID-19) still persists across the United States as mass vaccination is attempted using technologies new and old, without any publicly demonstrated cost-benefit analysis, lingering questions about short- and long-term safety both, and perhaps too late in the pandemic to achieve optimal results.1 There are still no approved drugs or drug combinations in the U.S. indicated for the ambulatory treatment of COVID-19 or its complications.
Unfortunately, there are no potentially conclusive randomized trials of early ambulatory multidrug therapy underway at this time. While some medical researchers announced promising treatments very early during the pandemic2,3—often multidrug therapeutic protocols administered as early as possible upon symptoms4—little attention has been paid to such results by the FDA or any other similar Western medical bodies.5 In particular, there are now numerous studies indicating the efficacy of hydroxychloroquine (HCQ) and ivermectin (IVM) in outpatient treatment or for mild COVID-19 patients who could be served by early ambulatory care.6,7
Doctors at All Valley Urgent Care (AVUC)—as well as others in the U.S. and around the world—used hydroxychloroquine within outpatient treatment protocols, providing the rationale for treatments examined in this paper. Later, ivermectin was added as a nearly uniformly positive body of evidence of its apparent efficacy was reported.8 Still, there is a vast pool of often uncollected or unpublished data regarding these treatments (personal correspondences).
As with all serious medical conditions, there is a role for empiric treatment in an attempt to reduce fatalities.9 This study provides a report that updates the totality of real-world data regarding multidrug protocols for the ambulatory care of a substantial number of patients with mild COVID-19, prior to progression to moderate or severe COVID-19 conditions.
We further summarize the logic behind emphasizing data within a picture of treatment optimization as opposed to a hierarchy of methodological preferences not tailored to the task. When waiting means losing lives to illness, rapid experimentation with safe, low-cost treatments is practical, economic science on a level that every physician can employ. Indeed, it is their ethical duty.
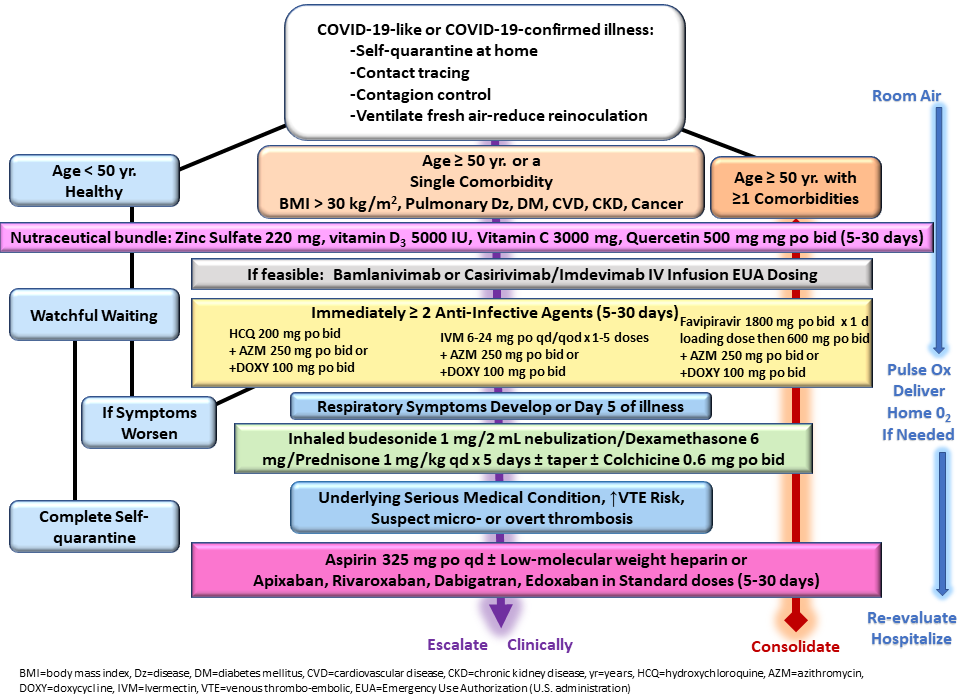
Risk stratification and advised nutraceuticals were in line with previously published guidance as shown in Figure 1,10 although physicians were allowed to exercise judgment. For instance, hydroxychloroquine or ivermectin were prescribed to patients deemed high risk who were PCR-positive prior to the development of any symptoms. All patients received empiric treatment on the first day of presentation in most cases before COVID-19 test results and treatment was continued for those with confirmed COVID-19. The protocols utilized agents with antiviral activity against SARS-CoV-2 (zinc, hydroxychloroquine, ivermectin) and one antibiotic (azithromycin, doxycycline, ceftriaxone), along with inhaled budesonide and/or intramuscular dexamethasone.
Albuterol nebulizer, inhaled budesonide, intravenous volume expansion with supplemental parenteral thiamine 500 mg, magnesium sulfate 4 grams, folic acid 1 gram, and vitamin B12 1 mg were administered for severely ill patients who either present or return to the clinic with severe symptoms.11 Additionally, for the severely ill population, dexamethasone 8 mg and ceftriaxone 1 gram was administered intramuscularly. A total of eight patients received monoclonal antibody treatment. All patients had in-person or telemedicine follow up at forty-eight hours, and as needed, depending on the duration and intensity of symptoms.12
Hospitalization and death data were collected on follow-up telemedicine visits or calls to family members. For the purpose of this paper, patients treated through October 21, 2020, were treated within Protocol 1, which includes all options listed above excluding ivermectin, and those treated October 22, 2020, through March 31, 2021, were treated within Protocol 2, which added ivermectin as an option.
2. Methods
2.1 Setting
Here, we report clinical outcomes associated with empiric multidrug regimens for confirmed COVID-19 patients who present to All Valley Urgent Care, which is a large, dedicated SARS-CoV-2 treatment center in El Centro, CA, between (Protocol 1) January 12, 2020, and October 21, 2020, and also (Protocol 2) between October 22, 2020, and March 13, 2021, endpoints inclusive. We compare the outcomes of the patients to which these protocols were applied against all 20,921 other known COVID-19 cases within Imperial County, CA during a similar time period (through May 3, 2021) where All Valley Urgent Care is located. Calculations for comparisons between groups and subgroups were performed using Excel or GraphPad Prism. Patient data for both All Valley Urgent Care and for Imperial County, CA was verified as received by the Public Health Department of Imperial County.
2.2 Inclusion-Exclusion and Categorization Criteria
Of the 4,385 COVID-19 patients recorded by Valley Urgent Care, a total of 3,962 treated patients were deemed to suffer from mild COVID-19 upon presentation, while 414 treated, but not immediately hospitalized patients had already progressed to moderate or severe COVID-19 stages of illness. One of those patients was treated for severe COVID-19, refusing immediate hospitalization. A total of nine patients were excluded due to nontreatment either because they were immediately sent to a hospital or because they refused treatment.
2.3 Confirmation of COVID-19 Diagnosis
Prior to May 15, 2020, patients were diagnosed with COVID-19 based on antibody-positive tests and symptom presentation per standard case definition guidance. From May 15, 2020, onward, patients underwent and tested positive using contemporary real-time polymerase chain reaction (PCR) assay tests from anterior nasal swab samples.
2.4 Patients
Among the 4,376 patients treated by All Valley Urgent Care staff, 2,137 (48.8%) were male, 2,239 (51.2%) were female, and 1,391 (31.8%) were fifty or older. Among these patients were 1,370 (31.3%) asymptomatic at presentation. A total of 1,980 (45.2%) patients received hydroxychloroquine, a total of 365 (8.3%) received ivermectin, while a total of 347 (7.9%) received both hydroxychloroquine and ivermectin.
2.5 Protocol Rationale
Prior to the COVID-19 pandemic, researchers noted the numerous general and specific qualities suggesting hydroxychloroquine’s potential to treat future coronavirus outbreaks.13-16 Shortly after the emergence of SARS-CoV-2, South Korea,17 and China18 rushed to a recommendation of hydroxychloroquine usage and a standard of care of the highly similar chloroquine drug in particular. Multiple rationale papers encouraged the research and medical communities to examine the effects of hydroxychloroquine.19-21
As hydroxychloroquine began to be used for prophylaxis or early ambulatory treatment in many nations such as India22,23 and Italy,24 it is noteworthy that its use grew in popularity rather than shrank. There seem to be no stories at all of doctors abandoning the use of hydroxychloroquine in outpatient care in the practice of empiric medicine, except where prohibited. This observation should not be viewed simply as evidence, but strong enough evidence to make it a priority for public health authorities to make it a commitment to turn anecdotes into working data.
Additionally, scores of studies have examined results from the use of hydroxychloroquine either as monotherapy or in multidrug regimens, as treatment for COVID-19 patients (listed at
https://c19hcq.com/
). We first note that many more of these studies reported more favorable results among patients receiving hydroxychloroquine, including conclusions in both categories (monotherapy or in a multidrug regimen) that did or did not reach statistical significance in isolation.25 While one such study can conceivably ascertain a high likelihood of causal reduction of disease progression or mortality through traditional tools of inferential statistics, some have claimed to demonstrate a lack of efficacy or even harm resulting from the use of hydroxychloroquine.
The latter form of conclusion results prima facia from the fallacy of assuming no other protocols exist that could improve the performance of the applied treatment. In fact, in fourteen out of fourteen published studies examining the mortality results of early treatment (largely at the mild stage prior to moderate disease progression) of COVID-19 patients with a moderate dose of hydroxychloroquine (no higher than 6g in total, but usually between 1.6g and 4.0g, spread out over several days), the treatment arm suffered lower mortality than the control arm.25 Logically, the success of any one treatment protocol represents the success of the medications used in that protocol. For instance, the results in Figure 2 represent a successful outcome, given a sufficiently powered sample size.
Further, there seems to be little if any effort to review the body of literature of the application of hydroxychloroquine, ivermectin, and other potentially effective therapeutics with the goal of optimizing outcomes that would, in a virtuous cycle, necessarily further tilt the summary of effects toward even greater efficacy of multidrug regimens such as the protocols we analyze in this study. By denying the value of empiric evidence and the experience of the doctors applying early ambulatory protocols to patients, health authorities miss the opportunity to collect valuable data and help those and other doctors network to share life-saving observations.
2.6 Analysis of Patient Outcomes by Protocol, Time Dependence, and Aggregation
The examination of the efficacy of potential antiviral agents requires progression-dependent stratification of results since antiviral effects are largest prior to the maturity of the viral replication process. For an antiviral drug effective in the treatment of COVID-19, results of patients treated early, at the mild disease state should be categorically different from the results of patients treated at the moderate disease stage, which may be further distinct from the results of patients treated after the onset of severe COVID-19.
Aggregating the results of treatments of a potential antiviral agent can dramatically alter effect sizes, and even produce results Simpson’s paradoxes (or more generally “Simpson’s effects” where data trends may not reverse, but appear dampened in magnitude), making effective treatments appear less helpful or even harmful. The skew in effect results after aggregation of patients of differing viral progression for an effective antiviral is clearly monotonic and negative, obscuring or reverse quantified effects.
The following hypothetical, but realistic charts demonstrate the need for stratification of results. In our hypothetical, the drug XYZ is assumed to reduce mortality by 60% when given to patients upon reaching Severity Score 4 of the WHO’s ordinal scale, as we see in direct protocol-level comparisons of Hospitals 1 and 3. However, a naïve analysis comparing patients who received drug XYZ to those who did not would conclude that drug XYZ results in a 37% relative increase in mortality, as demonstrated shown in Figure 3.
Often, studies attempt to correct such analysis in insufficient ways. For instance, grouping Hospitals 1 and 2 (those that use drug XYZ) against Hospital 3 would show a 40% reduction in mortality as opposed to the full 60% reduction in mortality.
Further, note that nothing in this hypothetical analysis implies anything about the efficacy of drug XYZ when given to patients earlier than at the point of hospitalization as should be optimal if drug XYZ has antiviral effects. There is no reason why, or any information that contradicts the possibility that drug XYZ, which seemed to do more harm than good in the original hypothetical analysis, could not effectively cure 100% of patients when applied very early. In particular, such results would still be consistent with an antiviral agent. While statisticians run results through Bayesian rubrics designed to correct for such flaws, such corrections may or may not come close to a perfect correction since, as we demonstrated, true optimal efficacy can still range anywhere from 60% to 100%. Stratifying results according to patient severity prevents these unnecessary flaws in the analysis.
Further, we suggest that much of the COVID-19 literature, most specifically that which examines effects of hydroxychloroquine as treatment, undergo re-analysis under a framework that separates analysis by protocol (specifically including severity stage at the time of treatment) rather than by sorting patients into binary categories defined by whether or not they received a particular drug without (enough) respect for the overall protocol. We also recommend that meta-analyses of hydroxychloroquine treatment results discontinue the inclusion of all studies that do not distinguish results at the protocol level, including stratification by the timing of treatment, and that studies included in such analyses necessarily be grouped according to stages of COVID-19 at which point treatment is delivered to each patient.
As we have heard the phrase, “garbage in, garbage out” applied to meta-analyses, so too should we apply it to studies such as the SOLIDARITY trials,26 and other randomized control trials’ (RCTs) use of hydroxychloroquine as monotherapy in exceptionally high and potentially dangerous doses on late-stage patients.27 Any conclusion that such an RCT could demonstrate the lack of efficacy of hydroxychloroquine in other protocols is non-sequitur and demonstrates that basic logic and critical thinking are, and have always been, the true gold standards of science.
Finally, we note that the form of retrospective observational analysis we present in this paper generates results nearly identical in nature and value to those of RCTs (using similar protocols).28,29 While it is technically conceivable that some unknown confounding variable both sorts the patients visiting Valley Urgent Care clinics from those in the general population and also affects disease progression, most such bias is likely corrected by the demographic risk analysis we apply in the following results—particularly given the recognized importance of age as a variable in predicting outcomes, along with the substantial, if imperfect, correlation between age and primary comorbidities. Most unknown confounding variables would also likely have been observed and documented at this point in a pandemic.
The number of patients in this study is large enough to lower the variance inherent in random sorting of unexamined risk factors as a proportion of sample size, and the effect sizes observed in this analysis are extremely large. As sample sizes grow large, the observed results of observational studies and RCTs tend to converge.28,29
2.7 Sensitivity Analysis
First and foremost, this study presents a large case series. We do our best to make the most of the results with comparison to both the local county case summary results, and synthetic versions of the county data. The primary limitation of our analysis is the synthetic comparator. In particular, nursing home patients who make up a substantial proportion of COVID-19 fatalities rarely overlap with patients seen at facilities such as ACUV. Our age mapping improves the comparison, but is still imperfect. In order to test the limitations, we compare the results of the treatment group to ideal cohorts under assumptions of varying hospitalization and mortality rates. The resulting confidence intervals suggest that even if imperfections in comparison warrant wider bounds, the resulting odds ratios are compelling.
3. Results
Given how few of the patients in Protocols 1 and 2 progressed to moderate or severe COVID-19, there was too little variation in results to confidently distinguish between the protocols on a statistical level, which could only be determined by extraordinarily large patient cohorts. This also means the positive results of this multidrug regimen can be more easily ascribed to hydroxychloroquine than to ivermectin, the primary hypothesized antiviral agents, though patients receiving ivermectin fared extremely well.
On the whole, the totality of effects can only be ascribed to the protocols as a whole, meaning there may be synergistic effects of some agents employed, or specific utility in the use of others (such as steroids). Each patient data set for Protocol 1, Protocol 2, and the combined patient aggregates showed dramatically lower rates of hospitalization and mortality relative to the general population in Imperial County, CA.
We note the limitations of retrospective observational studies with synthetic controls due to the lack of opportunity to sort at random or match them by propensity score, but believe that the glaringly small odds ratios, the combination of high patient numbers, and wide sensitivity analysis provide ample room for a declaration of significant positive results. Most certainly, the safety of numerous medications is demonstrated by the lack of serious adverse events for a substantial number of patients receiving each of hydroxychloroquine, ivermectin, azithromycin, doxycycline, albuterol, and budesonide.
The Valley Urgent care patient sets included fewer COVID-19 patients seventy or older (6.3%) than the larger county population (9.3%), and were more often male (48.8% vs. 47.4%). Mortality skewed heavily toward male COVID-19 sufferers in Imperial County. There is no way of knowing how many patients not treated at the Valley Urgent Care clinics also received similar forms of early ambulatory care including the medicines used in Protocols 1 and 2, so a comparison may result in dampened measures of relative risk for such outpatient treatment. Prior to computing p-values using Fisher’s Exact Test, we corrected for age using mortality factors implicit in the county-wide data (excluding patients in our Protocol 1 and 2 groupings).
Among 20,921 COVID-19 patients in Imperial County, CA who were not treated by Valley Urgent Care, 4,770 (22.8%) were hospitalized and 636 died (3.0%).
Of 1,585 patients treated for mild COVID-19 in Protocol 1, there was one hospitalization (0.06%) and zero deaths (0%). Of 2,356 patients treated for mild COVID-19 in Protocol 2, there was one hospitalization (0.04%) and zero deaths (0%). There were twenty-one patients whose date of treatment was obscured after data blinding, but were treated for mild COVID-19, all without hospitalization or death. In total, of 3,962 patients (Table 1) treated for mild COVID-19 by All Valley Urgent Care staff, prior to moderate or severe disease progression, there were two hospitalizations (0.05%, RR = 0.0019, p < 0.0001) and zero deaths (0%, RR = 0.00, p < 0.0001).
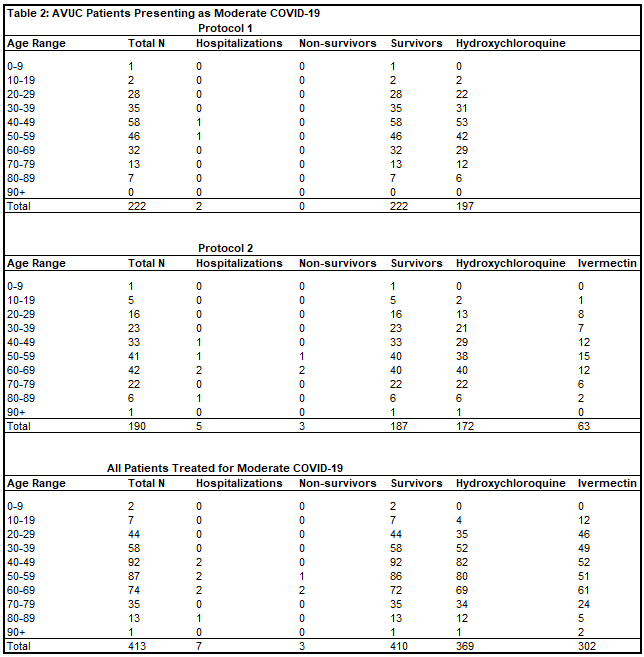
Of 413 patients treated for moderate COVID-19 in Protocol 1, there were two hospitalizations (0.5%) and zero deaths (0%). Of 190 patients treated for moderate COVID-19 in Protocol 2, there were five hospitalizations (2.6%) and three deaths (1.6%). There was one patient treated whose date of treatment was obscured after data blinding, but was treated for moderate COVID-19 without hospitalization or death. In total, of 413 patients (Table 2) treated for moderate COVID-19 by Valley Urgent Care, there were seven hospitalizations (1.7%) and three deaths (OR = 0.0659, p < 0.0001). The one patient who refused hospitalization, and instead chose outpatient treatment for severe COVID-19 with All Valley Urgent Care recovered.
Those receiving treatment while presenting mild COVID-19 were less often hospitalized (OR = 0.0293, p < 0.0001) and suffered lower mortality (OR = 0.0000, p = 0.0008).
To understand the limitations of comparison, we ran a sensitivity analysis. Comparison groups included Imperial County’s data through May 15, 2021, a version of that cohort with age profiles adjusted (corrected) to match the All Valley Urgent Care patient profiles, and several cohorts with decreasing hospitalization and mortality. Finally, we computed the lower bounds of hospitalization and mortality numbers for which the All Valley Urgent Care showed statistically significant improvement, which were a 0.20% hospitalization rate and a 0.10% case fatality rate (Table 3).
We note here that corrections for comorbidities or symptoms is both impossible and unnecessary for this analysis. It is impossible because such information is rarely gathered prior to the point of hospitalization, which is nearly always the demarcation point between mild and moderate COVID-19. These other factors are also highly correlated with age distribution, and with such a large sample size, it is doubtful that the data relationships change substantially.
4. Discussion
A handful of analyses have been published on early ambulatory care of COVID-19 patients. While almost uniformly positive with respect to hydroxychloroquine and ivermectin specifically, as well as smaller numbers of studies on fluvoxamine, proxalutamide, bromhexine, and other drugs, there is a vast amount of still uncollected data on these and numerous other treatments.
Such data collection should always have been a high priority for health officials and the larger medical community. It should have been a national priority. Going forward, it should be entirely unacceptable that any organization discourages the collection, organization, examination, and analysis of empiric treatment regimens developed by caring, diligent, and networked health professionals. To the extent that public health authorities operate, it should be inherent in their duties to encourage if not participate in that process as a primary duty.
We believe that that the case for early ambulatory care for COVID-19 patients utilizing multidrug regimens such as those described in this paper, using hydroxychloroquine, ivermectin, or potentially improvements on these options, has been amply demonstrated. The collection of unexamined pools of data, analyzed on a similar protocol level, will further demonstrate the case for early ambulatory treatment, and in particular, using multidrug regimens under similar or other protocols. Optimization of medical treatment cannot fully take place until these results are recognized, either by health officials, if not by the larger community of physicians capable of delivering these treatments or their patients.
It is difficult to imagine that optimization would involve the current standard of leaving patients untreated until the development of moderate or severe COVID-19 symptoms. All pertinent data suggests otherwise.
5. Conclusion
Our study found that early ambulatory treatment for SARS-CoV-2 infection and resulting COVID-19 disease is extremely safe, feasible, practical, and scalable to large numbers of patients. It is extremely important to encourage early ambulatory treatment, both among patients and doctors alike. These results demonstrate that hospitalization and death were nearly nonexistent when patients were treated with multidrug regimens in protocols that included hydroxychloroquine and ivermectin prior to progression beyond the mild COVID-19 disease state.
Similar therapies also showed statistically significant reductions in hospitalization and death among those treated during the moderate COVID-19 disease state. Given the depth of the COVID-19 crisis and the high mortality rate for hospital-initiated treatment, we conclude that early ambulatory multi-drug therapy should be a standard of care for high-risk patients. It is no longer tenable to delay treatment until a hospitalization for patients who can easily be treated early as outpatients with a well-managed protocol.
Acknowledgments
The authors thank all of the doctors and medical staff at the All Valley Urgent Care clinics, the Public Health Department of Imperial County, CA, as well as the maintainers of the Imperial County COVID-19 data and associated tracking systems. We thank all of the doctors and researchers who have thoughtfully generated so much useful research during the pandemic, and particularly those whose insights into early ambulatory treatment protocols encouraged the practice of valuable care at All Valley Urgent Care and this analysis of the data.
Funding
This study was entirely self-funded.
Competing Interests
None of the authors reports any conflicts of interest.
Bibliography for Study
1. McCullough PA, Eidt J, Rangaswami J, et al. "Urgent need for individual mobile phone and institutional reporting of at home, hospitalized, and intensive care unit cases of SARS-CoV-2 (COVID-19) infection." Rev Cardiovasc Med 2020; 21(1): 1-7.
2. Gautret P, Lagier JC, Parola P, et al. "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial." Int J Antimicrob Agents 2020; 56(1): 105949.
3. Risch HA. "Early Outpatient Treatment of Symptomatic, High-Risk COVID-19 Patients That Should Be Ramped Up Immediately as Key to the Pandemic Crisis." American Journal of Epidemiology 2020; 189(11): 1218-26.
4. Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA. "Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection." Rev Cardiovasc Med 2020; 21(4): 611-4.
5. Derwand R, Scholz M, Zelenko V. "COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study." International Journal of Antimicrobial Agents 2020; 56(6): 106214.
6. Ip A, Ahn J, Zhou Y, et al. "Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study." BMC Infectious Diseases 2021; 21(1): 72.
7. Guérin V, Lévy, P., Thomas, J.-L., Lardenois, T., Lacrosse, P., Sarrazin, E., Andreis, N. R.- de, & Wonner, M. "Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19." Asian Journal of Medicine and Health 2020; 18(7): 45-55.
8. Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. "Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19." Am J Ther 2021; 28(3): e299-e318.
9. McCullough PA, Oskoui R. "Early multidrug regimens in new potentially fatal medical problems." Rev Cardiovasc Med 2020; 21(4): 507-8.
10. McCullough P. "Innovative Early Sequenced Multidrug Therapy for Sars-Cov-2 (Covid-19) Infection to Reduce Hospitalization and Death." International Journal of Medical Science and Clinical Invention 2020; 7(12): 5139-50.
11. Flannery AH, Adkins DA, Cook AM. "Unpeeling the Evidence for the Banana Bag: Evidence-Based Recommendations for the Management of Alcohol-Associated Vitamin and Electrolyte Deficiencies in the ICU." Crit Care Med 2016; 44(8): 1545-52.
12. Colbert GB, Venegas-Vera AV, Lerma EV. "Utility of telemedicine in the COVID-19 era." Rev Cardiovasc Med 2020; 21(4): 583-7.
13. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. "Effects of chloroquine on viral infections: an old drug against today's diseases?" Lancet Infect Dis 2003; 3(11): 722-7.
14. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. "In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine." Biochem Biophys Res Commun 2004; 323(1): 264-8.
15. Vincent MJ, Bergeron E, Benjannet S, et al. "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread." Virology Journal 2005; 2(1): 69.
16. Rolain JM, Colson P, Raoult D. "Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century." Int J Antimicrob Agents 2007; 30(4): 297-308.
17. Sung-sun K. "Physicians work out treatment guidelines for coronavirus." 2.13.2020 2020. http://www.koreabiomed.com/news/articleView.html?idxno=7428.
18. Gao J, Tian Z, Yang X. Breakthrough: "Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies." Biosci Trends 2020; 14(1): 72-3.
19. Vetter P, Eckerle I, Kaiser L. "Covid-19: a puzzle with many missing pieces." BMJ 2020; 368: m627.
20. Pang J, Wang MX, Ang IYH, et al. "Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review." Journal of Clinical Medicine 2020; 9(3): 623.
21. Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. "Chloroquine and hydroxychloroquine as available weapons to fight COVID-19." Int J Antimicrob Agents 2020; 55(4): 105932.
22. Chatterjee P, Anand T, Singh K, et al. Healthcare workers & SARS-CoV-2 infection in India: "A case-control investigation in the time of COVID-19." Indian Journal of Medical Research 2020; 151(5): 459-67.
23. Bhattacharya R, Chowdhury S, Nandi A, et al. "Pre-exposure hydroxychloroquine prophylaxis for COVID-19 in healthcare workers: a retrospective cohort." 2020 2020; 9(1): 8.
24. Berardi F. "The Italian Doctor Flattening the Curve by Treating COVID-19 Patients in Their Homes." TIME. 2020 April 9, 2020.
25. hcqmeta.com. "HCQ for COVID-19: real-time meta analysis of 265 studies." 2021 (accessed July 19, 2021.
26. Consortium WST. "Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results." New England Journal of Medicine 2020; 384(6): 497-511.
27. Group RC. "Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19." New England Journal of Medicine 2020; 383(21): 2030-40.
28. Concato J, Shah N, Horwitz RI. "Randomized, Controlled Trials, Observational Studies, and the Hierarchy of Research Designs." New England Journal of Medicine 2000; 342(25): 1887-92.
29. Krauss A. "Why all randomised controlled trials produce biased results." Annals of Medicine 2018; 50(4): 312-22.





Comments
Post a Comment