SCIENTISTS USE MRNA TECHNOLOGY TO CREATE A POTENT FLU VACCINE THAT COULD LAST FOR YEARS
IN THE SPRING OF 1933, three scientists at the U.K.’s Medical Research Council received a treasure trove of vials containing human mucus from sick hospital patients. It was gross, yes, but not unwarranted: The culprit behind what had caused the 1918 influenza pandemic was still at large.
Months later, the trio of scientists published a paper that found that a virus, not a novel strain of bacteria like some within the scientific community originally thought, was to blame. Over the following decades, other scientists unfurled the gnarly branches of the large influenza family tree, gathering enough information to formulate a vaccine, which (hopefully) most of us get before every flu season.
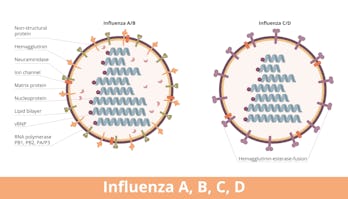
But here’s the catch: Influenza is a master shapeshifter. Every year, strains of the virus that infect humans — influenza type A and B — evolve in ways that evade vaccines and, subsequently, our immune systems. This results in uneven vaccine effectiveness from year to year and also undermines efforts to pack a flu shot with a broad, long-lasting immune punch.
But we may have an ace in the hole thanks to mRNA, the same technology used for our Covid-19 vaccines. In a study published Monday in the journal Proceedings of the National Academy of Sciences, researchers at the University of Pennsylvania, Icahn School of Medicine at Mount Sinai, and other institutions have cooked up an mRNA-based influenza vaccine that targets four viral proteins that tend to remain the same across different strains of influenza.
“[It’s] a nice study with significant clinical implications that uses the same strategy as for Covid vaccination to create a better flu vaccine with broader capabilities by targeting multiple conserved flu [viral proteins] antigens instead of the way we do it now, which is to grow candidate strains in eggs and then inactivate them,” Anne Davidson, a researcher at the Feinstein Institutes for Medical Research in New York, who was not involved in the study, told Inverse in an email.

HERE’S THE BACKGROUND — Flu vaccines are updated every year based on influenza patterns most recently seen in the southern hemisphere, such as Australia; its flu season runs from April to October.
Scientists pay close attention to one sugar-covered protein called hemagglutinin that dots the surface of the influenza virus, Norbert Pardi, an assistant professor of microbiology at the University of Pennsylvania Perelman School of Medicine who co-led the study, tells Inverse.
“Hemagglutinin has two major parts: the head domain, which is variable and immunodominant, [and a stalk domain],” explains Pardi. “The current seasonal vaccines that use three or four inactivated [influenza] viruses primarily target the immunodominant head domain… but the problem is that the virus can change that pretty easily and escape from protective immunity.”
A better option would be to target viral proteins that don’t switch up and stay pretty much the same regardless of which strain of influenza you’re infected with, says Pardi and Florian Krammer, a virologist at the Icahn School of Medicine at Mount Sinai who also co-led the study.
Those proteins include neuraminidase, nucleoprotein, and matrix protein 2, all of which help the virus make copies of itself.
Krammer says while there have been efforts over the years to include conserved influenza proteins, such studies have run up against failure and a whole host of hurdles. For example, to measure a potential shot’s effectiveness, scientists look to see whether it triggers an immune response, indicated via the presence of antibodies.
“The vaccines that we’re testing these proteins with, they don’t induce those types of antibodies that are used as correlates,” explains Krammer. “So there’s not a lot of confidence during clinical development.”
HOW THEY DID IT — What has really made the dream of a potent flu shot come alive, says Pardi and Krammer, is messenger RNA (or mRNA) technology, the same used for Moderna and Pfizer-BioNTech’s Covid-19 vaccines. It involves taking a piece of genetic code that isn’t capable of altering anyone’s DNA and instructing it to make a particular type of protein that the immune system can then learn to recognize and target.
For their influenza vaccine, the researchers created an mRNA cocktail encoding the four influenza proteins neuraminidase, nucleoprotein, matrix protein 2, and the stalk portion of hemagglutinin (which is conserved compared to its head domain).
The vaccine was then injected into a group of twenty or so naive mice who had never experienced influenza before. They either got a quadrivalent jab (meaning all four mRNA segments for each protein was present) or monovalent (the conventional flu vaccine or vaccines containing an individual mRNA for any one of the proteins). Some animals received one shot, while others lucked out with one shot plus a booster four weeks later. The mice were then challenged with an assortment of different influenza strains, both that infect humans and other animals like dogs.
WHAT THEY FOUND — There were two important phenomena the researchers noticed. First, while both the quadrivalent and monovalent jabs encouraged antibody production, only the quadrivalent shot protected the mice against the viral challenge, with exception of the monovalent vaccine containing nucleoprotein, which seemed to protect vaxxed mice from death.
While antibodies tend to hog the vaccine limelight, immune cells called T cells, which roam the body and kill infected cells, are also crucial in the fight against viruses. Pardi, Krammer, and their colleagues found that the nucleoprotein-specific jab encouraged a class of cytotoxic T cells that studies have found play an important role in protection against severe influenza infection in humans and animal models.
Krammer says this motley of immune responses to the four different influenza viral proteins suggests it's more powerful to have a colorful vaccine cocktail rather than a drab monotone.
“When we mix all of them together, we get the broadest immune response,” he says. “You get the engagement of T cells against the nucleoprotein, you get antibodies, and we get a pretty strong neuraminidase response. That’s kind of the beauty here that you’re flexible in what types of [viral proteins] you use… and you have a lot of possibilities to try [out].”
The researchers also expect it wouldn’t need to undergo annual updates as our current ones do. Instead, they might last for a few years.
WHAT’S NEXT — While mice studies are the launch point for future studies into other organisms like monkeys and eventually humans, Pardi and Krammer say it’s not clear whether what we see in mice will necessarily translate to us. For one, humans don’t have a naive immune system like the mice used in the study. Our pre-existing immunity against the flu may impact the quality of any potential vaccine’s antibody response, according to some studies.
“Clinical trials will be necessary to see how vaccines can overcome the issue with the shortfall from previous immunity,” says Pardi. “Pretty much all adults have antibodies against the flu, and we need to see how our vaccine can overcome this issue.”
It may take several years of research and clinical trials before their mRNA influenza vaccine sees the light of day, say Pardi and Krammer. In the meanwhile, there’s no point waiting: Go get your flu shot, dear reader.
****************
Another FUN moment... Enjoy!
Comments
Post a Comment